This is a sling that re-locates the urinary sphincter mechanism to facilitate better control of the passage of urine from the bladder – it restores continence, and offers an alternative to pads, catheters and artificial sphincter as a treatment for urinary incontinence.
What happens during the procedure?
-
You will receive a full general anaesthetic.
-
You may also receive an epidural (spinal) as well.
-
You will be given intravenous antibiotics before and after the procedure.
-
You will have a small incision between the scrotum and the anus (the perineum) to allow the sling to be positioned to elevate the urethra.
-
Two small second incisions are made in the groin to allow appropriate positioning & tensioning of the sling.
-
A urinary catheter will be inserted into the bladder and will remain in place for 24 hours.
Components
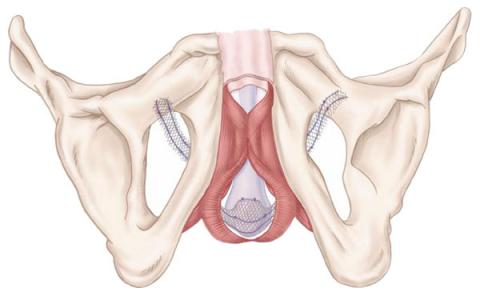
The AdVance® Male Sling is a synthetic sling designed to sit under the urethra to support & elevate the urinary sphincter to a position to allow it to function in a normal manner, thus restoring urinary continence.
It consists of two components or sections:
-
Sling – this is the broad pre-cut mesh component that sits under the urethra providing the necessary support.
-
Arms – these extend laterally from the central sling component and “wrap” around the obturator foramen to allow positioning and elevation of the AdVance sling to the most effective situation.
Side Effects
All operations have the potential for side effects. Most people do not experience any (< 10% of patients actually do), though it is good to be aware of what they might be:
Occasional - < 10%
-
Burning or blood when you pass urine - usually settles in a few days.
-
Temporary insertion of catheter for urinary retention.
-
Temporary perineal pain.
Uncommon - < 5%
-
Frequency & urgency when passing urine - Overactive bladder symptoms (OAB).
-
Urine infection.
Rare - < 1%
-
Wound infection.
-
Erosion of sling exposing the device to urine - <0.01%.
